Last January 17, 2018, we did our IBT on chemical energetics. We were given eleven substances namely hydrochloric acid, sodium hydroxide, zinc dust, barium chloride, ammonium chloride, sodium hydroxide flakes, magnesium nitrate, potassium dichromate, ethanol, and anhydrous copper sulfate. By performing certain procedures, we were asked to identify each reaction as either endothermic or exothermic through temperature reading. In order for us to determine the changes in temperature, we took note of the initial temperature reading of the first solution or water and then, the final temperature reading.
The following were the results we obtained:
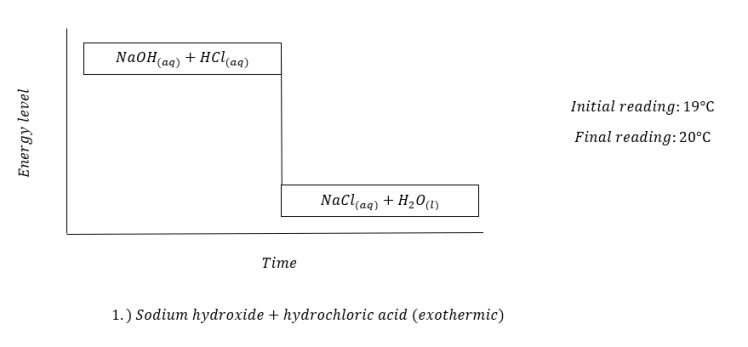

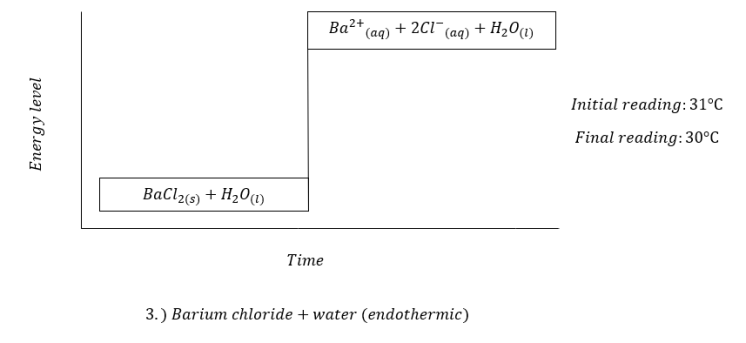


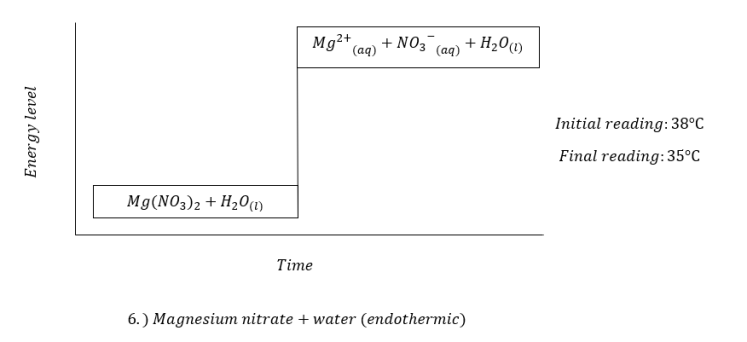


This experiment made me recognize the full worth of different reactions. Different substances can form reactions that our naked eye can not see. We can only determine these changes by using certain materials which can only be found in a laboratory.
While the temperature of the solutions changed, my knowledge on these substances grew more and I think that’s the beauty of this experiment.
This experiment was fun and challenging. Even though Chemistry is already done, I’m still looking forward to experiments like this in the future. 🙂